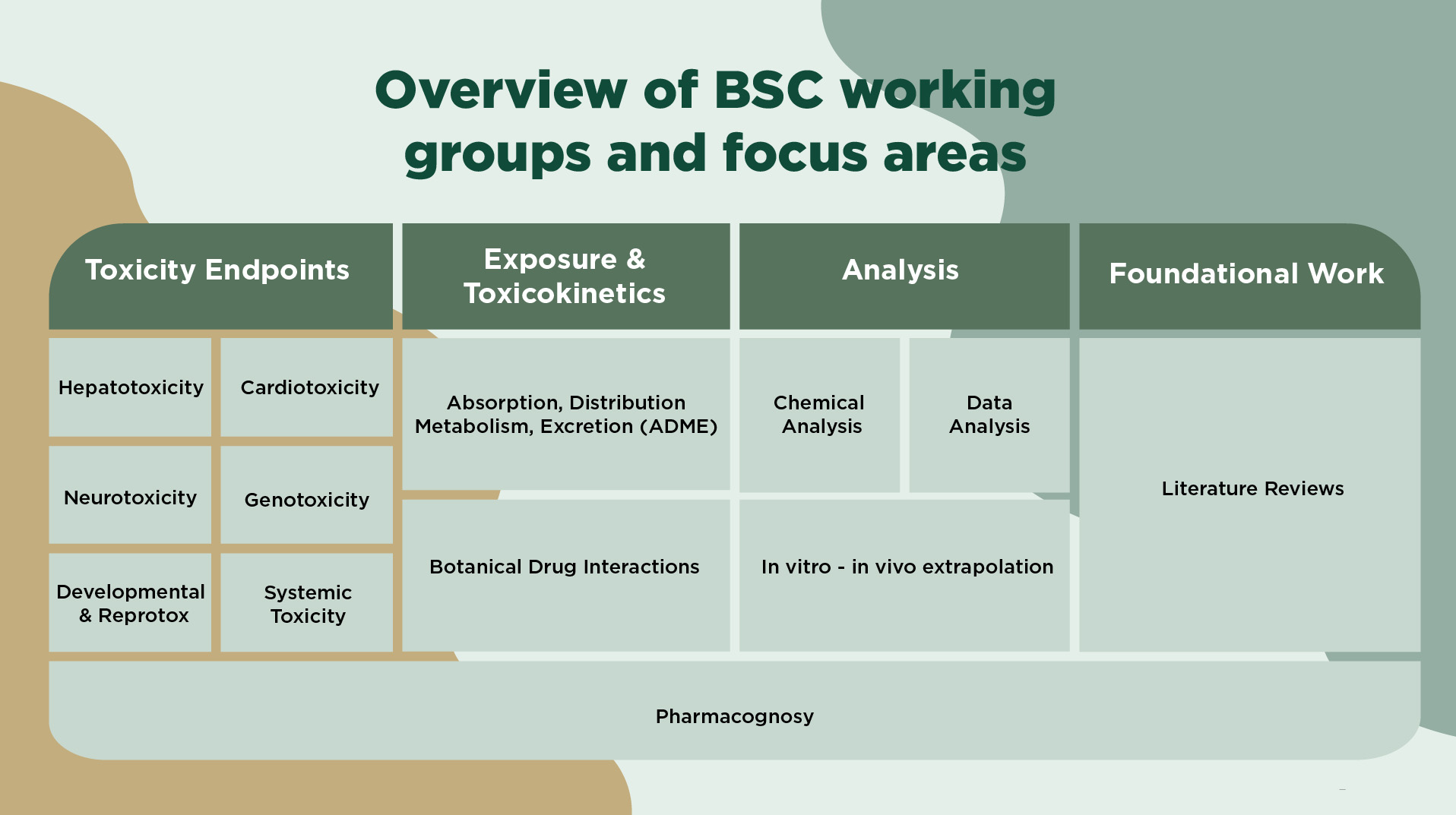
Botanical Safety Consortium Technical Working Groups
The BSC's Technical Working Groups (TWGs) are the core technical entities responsible for designing and executing the BSC’s scientific mission.
They are focused on specific scientific questions related to evaluation of botanical ingredient safety, as defined by each TWG’s mission and objectives.
Being part of a TWG involves:
- Participating in teleconferences
- Providing technical and strategic input and perspectives during the TWG’s ongoing scientific discussions
- Experimental research
- Data analysis
- Writing
- Communicating on behalf of the TWG
Sub-teams within a TWG may be formed as needed on key topics related to the overall TWG mission & objectives.
- TWG members may be nominated by BSC Steering Committee, Stakeholder Council members, TWG members, themselves, or staff.
- Eligibility for TWG participation will be evaluated by the BSC Steering Committee and TWG co-chairs based on scientific and technical expertise via submission of an application and CV.
- As is feasible, all TWGs will strive for balance across sectors (public / private), areas of expertise, and geography.
- Members of the TWG will review membership on a yearly basis
Chemical Analysis
Mission: To develop a strategy and methodologies to characterize botanical ingredients for the purpose of enabling safety assessments.
Objectives:
- Prioritize selected candidates for comprehensive chemical characterization based on the needs of other Technical Working Groups;
- Identify a strategy to compile existing literature on analytical methods used and chemical composition of selected botanical;
- Select resource-efficient analytical approaches, methods, and partners that can comprehensively characterize botanical ingredients with respect to safety, including, but not limited to, identifying and quantifying constituents of botanicals to the degree required for material selection and safety assessment.

Developmental and Reproductive Toxicity (DART)
Mission: To develop screening strategies that can reliably identify potential developmentally or reproductively toxic botanicals.
Objectives:
- Select in silico and in vitro tools that can accommodate complex mixtures represented by botanicals;
- Select candidate botanicals based on suspected toxicity or safety with respect to DART endpoints;
- Establish a series of DART botanical case studies, such that we can evaluate the usefulness of a growing toolbox.
Hepatotoxicity
Mission: To develop a screening strategy that can reliably identify hepatotoxic botanicals, inform mechanisms of toxicity, and characterize the ‘botanicokinetic’ properties of botanical ingredients.
Objectives:
- Select resource-efficient in silico and in vitro tools that can accommodate complex mixtures represented by botanicals. These tools will estimate human liver potential or ADME properties;
- Select candidate botanicals with respect to hepatotoxicity based on suspected toxicity or safety, and ADME endpoints;
- Evaluate the potential and limitations of these tools to predict and understand botanical-induced hepatotoxicity;
- Explore the potential of drug-botanical interactions using candidate methods.

Genotoxicity

Mission: To develop a screening strategy that can reliably identify potential genotoxic botanical ingredients, with future application to evaluate associated human health risks.
Objectives:
- Select resource-efficient in silico and in vitro tools that can identify genotoxic agents in complex mixtures represented by botanicals;
- Recommend criteria for identifying significant genotoxic hazards;
- Select candidate botanicals based on suspected toxicity or safety with respect to genotoxicity endpoints;
- Use a series of botanical case studies to evaluate the usefulness and reliability of a growing genotoxicity toolbox in the context of in vivo outcomes and exposure (where such data are available).
Systemic Toxicity
Mission: To develop tools that can reliably identify botanicals with the potential to induce adverse effects within multicompartmental biological systems.
Objectives:
- Develop systematic literature review strategies and tools to efficiently gather existing history of use and traditional use data for botanicals.
- Select and leverage multi-compartmental in vitro models to generate systemic safety data for botanicals
- Improve in vitro to in vivo extrapolation (IVIVE) using toxicokinetic modeling to support safety assessments and margin of safety calculations

Data Analysis

Mission: To develop and apply data analytic methods that use in vitro/in silico data for safety/hazard assessment of botanicals.
Objectives:
- Work with other Technical Working Groups to design experiments;
- Support the data analysis needs of other Technical Working Groups;
- Development, evaluation and/or application of data analysis methods for the determination of botanical sufficient similarity;
- For a subset of botanical, characterize the degree of similarity between the toxicological profiles derived from in vitro/in silico data compared to those obtained from traditional animal tests
Cardiotoxicity
Mission: To develop screening strategies that can reliably identify potential cardiotoxic botanicals.
Objectives:
- Select in silico and in vitro tools that can accommodate complex mixtures represented by botanicals
- Select candidate botanicals based on suspected toxicity or safety with respect to cardiotoxicity endpoints
- Establish a series of cardiotoxicity botanical case studies, such that we can evaluate the usefulness of a growing toolbox

Neurotoxicity
Mission: To develop screening strategies that can reliably identify potential neurotoxic botanicals.
Objectives:
- Select in silico and in vitro tools that can accommodate complex mixtures represented by botanicals
- Select candidate botanicals based on suspected toxicity or safety with respect to neurotoxicity endpoints
- Establish a series of neurotoxicity botanical case studies, such that we can evaluate the usefulness of a growing toolbox
Pharmacognosy
This group is comprised of experts who advise the BSC on an ad-hoc basis on botanical candidates and sources of available botanical information. They also review compiled data and literature produced by the BSC and recommend additional stakeholders to inform the consortium in key areas.