BSC Newsletter - December 2020
The Botanical Safety Consortium (BSC) was officially convened in November 2019 as the result of a Memorandum of Understanding (MOU) between the US Food and Drug Administration (FDA), the National Institute of Health’s National Institute of Environmental Health Sciences (NIEHS), and the non-profit Health and Environmental Sciences Institute (HESI). The BSC is a multi-partite, multi-stakeholder, international effort that brings together key scientific experts to enhance the botanical safety toolkit and bring clarity to botanical dietary ingredient assessments. It was formed to provide a sound scientific basis for integrating existing botanical safety and toxicity information with the latest toxicological tools.
The objectives of the BSC are:
- To engage with a broad group of global stakeholders to leverage the best scientific safety approaches;
- To establish the appropriate levels of chemical characterization for complex botanical ingredients;
- To identify pragmatic, fit-for-purpose in vitro and in silico assays to evaluate botanical safety;
- To evaluate the application of these tools via comparison to the currently available safety information;
- And to integrate these tools and approaches into a framework that can facilitate robust evaluation of botanical ingredients.
Save the date for the following BSC events!
- Society of Toxicology (SOT) Session 2021: Botanical Mixtures: Predictive Approaches to Evaluating Pregnancy and Reproductive and Developmental Health
Date: Wednesday, 24 March 2021
Time: 11:45 AM to 2:30 PM EST
- Congress of Toxicology in Developing Countries (CTDC11) Keynote Talk: The Botanical Safety Consortium: A Public-Private Partnership to Enhance the Botanical Safety Toolkit
Date: Tuesday, 15 June 2021
- 11th World Congress on Alternatives and Animal Use in the Life Sciences Poster: The Botanical Safety Consortium’s Strategy for Developing A Robust Framework Of Genotoxicity Assays For Safety Assessment Of Botanical Substances
Date: Sunday, 22 August 2021 - Thursday, 26 August 2021
For any organization interested in joining the BSC, there are multiple levels of involvement depending on interest and expertise. The general public can keep up to date by visiting our website at botanicalsafetyconsortium.org.
The Stakeholder Council is comprised of members with interest and expertise in botanical ingredients, supplements, or the technologies involved. Members of the Steering Committee and Technical Working Groups are also members of the Stakeholder Council. Stakeholders participate by:
- Voting on Steering Committee composition via electronic ballot
- If nominated and elected, serving on the Steering Committee
- Participating in Stakeholder Council technical update webinars
- Attending the BSC Annual Meeting
- Submitting proposals for new areas of scientific work by the BSC
- Responding to specific requests for input from the Steering Committee and/or Technical Working Groups related to the ongoing BSC work
- Recruiting additional public or private participants to join the efforts and mission of the BSC
- If contacted, assisting in providing feedback and reviews on research, communications, etc., based on expertise categories
The Technical Working Groups are made up of scientists with specific technical expertise related to the specific topic of the working group. Technical Working Group members directly contribute to the strategy and science of the BSC. Being on a Technical Working Group involves some, but not all, of the following in addition to the points above listed for the Stakeholder Council:
- Participating in teleconferences
- Providing technical and strategic input and perspectives during scientific discussions
- Experimental research
- Data analysis
- Manuscript preparation
- Communicating on behalf of the Technical Working Group
Joining the Stakeholder Council or a Technical Working Group from the private sector requires a financial contribution. More information can be found here.
The Technical Working Groups in the BSC are the following:
- Cardiotoxicity
- Chemical Analysis
- Data Analysis
- Developmental and Reproductive Toxicity (DART)
- Genotoxicity
- Hepatotoxicity/ADME
- Neurotoxicity
- Systemic Toxicity/IVIVE
Each of the toxicological technical working groups have selected botanical ingredients with existing safety or toxicity data related to their endpoint as case studies. Groups are now selecting assays that can be used as screening tools to test their suitability for botanical ingredients as complex mixtures. The Systemic Toxicity Technical Working Group is also creating a template for a fit-for-purpose literature review to aid the other groups. Additionally, there is a Pharmacognosy Group that serves in an advisory role for the groups.
The Chemical Analysis Technical Working Group has created a workflow for botanical authentication and constituent identification. The Data Analysis Technical Working Group is creating a data template that the toxicological groups can utilize.
Next year, we will begin work with our pilot botanical ingredient (ashwagandha root extract) for chemical analyses and a literature review, followed by in silico studies for the different endpoint groups, which will be followed by in vitro tests.
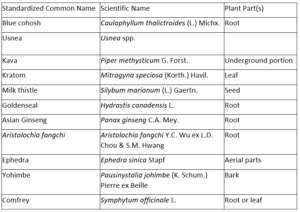
Below is a table of botanical ingredients we plan to source next. We are not making safety determinations on these botanical ingredients. We are working to see if the data we generate with non-mammalian toxicity tests will compare to existing data from animals or humans to see if our toolkit is useful. Botanical ingredients on this list are not necessarily used as dietary supplements. Ingredients below were selected by the BSC based on existing safety or toxicity data.
If you know a reputable supplier for any of these botanical ingredients, please contact Connie Mitchell (cmitchell@hesiglobal.org) or the BSC (botanicalsafety@hesiglobal.org).