New publication in Regulatory Toxicology and Pharmacology
Improving the rigor and utility of botanical toxicity studies: Recommended resources
Deval Patel, Barbara C. Sorkin, Constance A. Mitchell, Michelle R. Embry, Sharline Rina-Kong, Rebecca E. Adams, Emily R. DeTemple, Aalekhya Reddam, Stefan Gafner, Olaf Kelber, Cynthia V. Rider, Hellen Oketch-Rabah, Amy L. Roe, Robin J. Marles, Joseph Dever, Steven Dentali. Regulatory Toxicology and Pharmacology. https://doi.org/10.1016/j.yrtph.2023.105471
The use of botanicals in dietary supplements and other products is increasing. This article and template provide a way for researchers to gather information for studying the toxicity of these natural products in order to improve the quality of data being generated. It outlines resources to collect literature and relevant information to support the design of botanical toxicity studies. These resources provide critical information related to botanical identification, characterization, pre-clinical and clinical data, including adverse effects and interactions with pharmaceuticals.
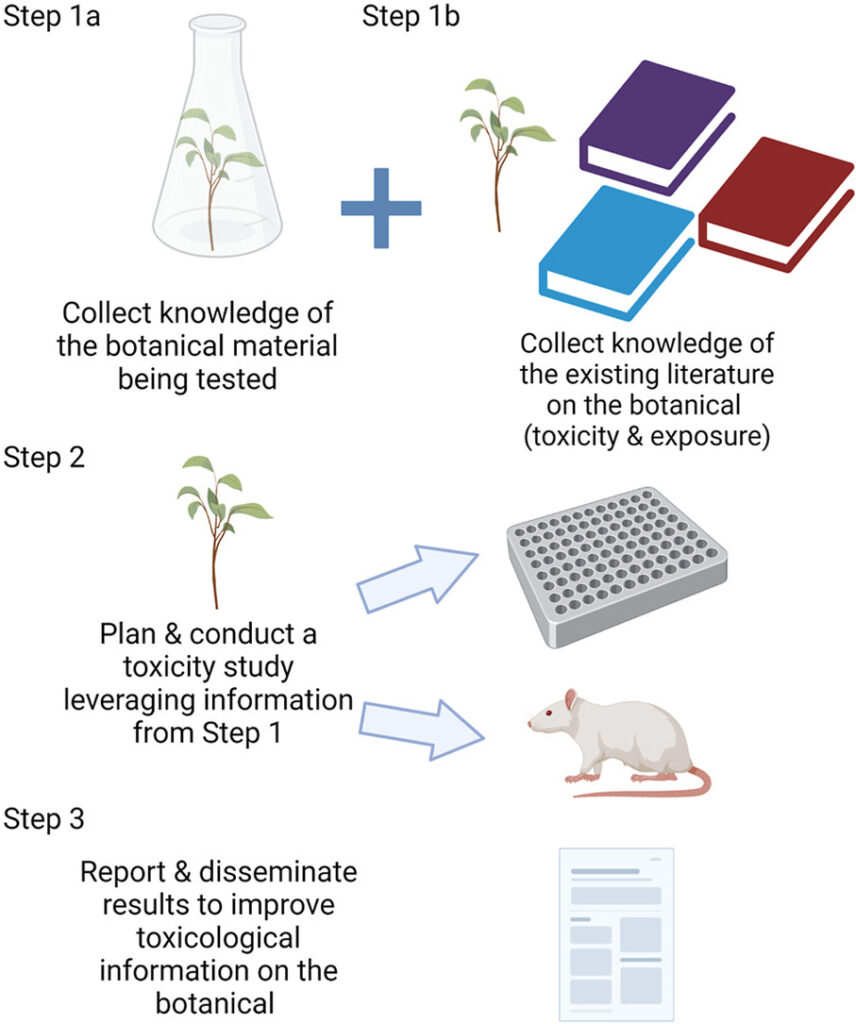
Graphical abstract from ScienceDirect
