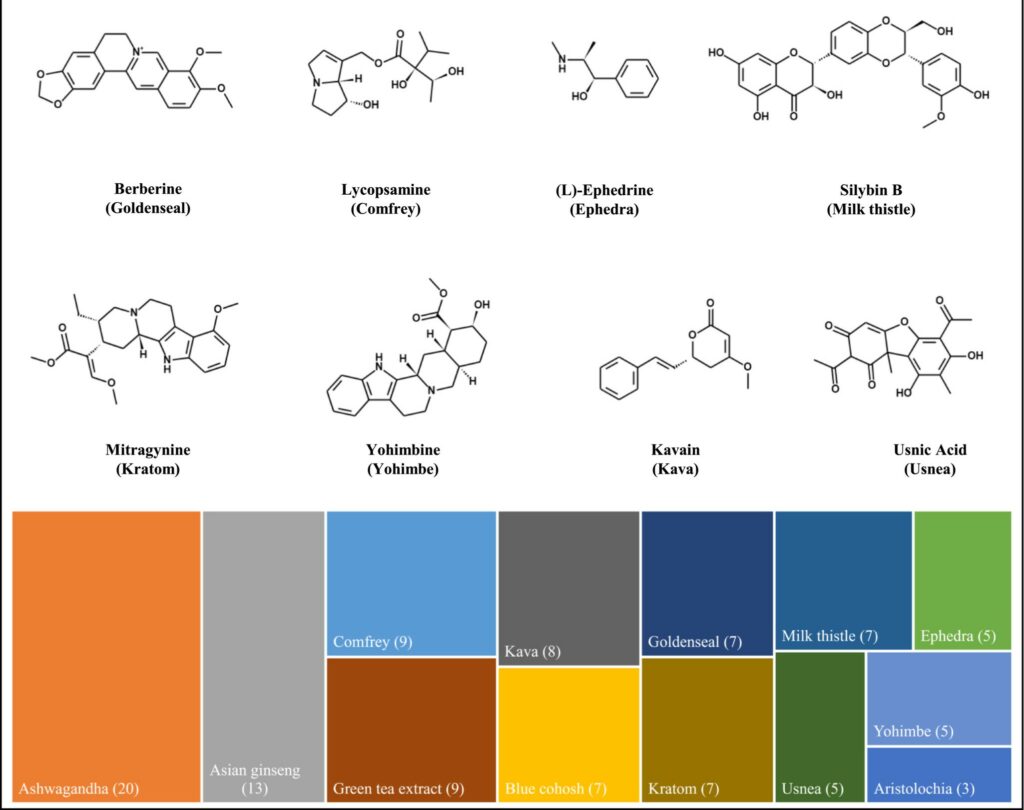
New publication: Prediction of physicochemical and pharmacokinetic properties of botanical constituents by computational models
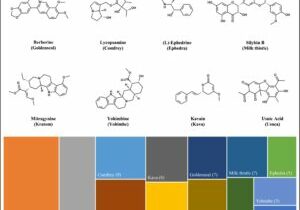
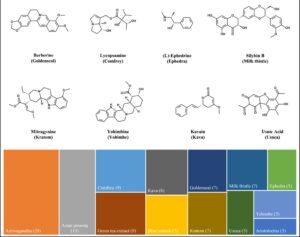
A newly published study from the Botanical Safety Consortium used computational models to predict the physiochemical and pharmacokinetic properties of botanical constituents. This study investigated 103 major compounds from 13 botanicals (e.g., ashwagandha, kratom, and yohimbe), providing insights into their absorption, bioavailability, and safety profiles. The in-silico predictions could help guide future safety studies and the…
New publication: Within-laboratory reproducibility of Ames test results: Are repeat tests necessary?
A cross-sector group of experts from the Health and Environmental Sciences Institutes (HESI)’s Botanical Safety Consortium (BSC) and Genetic Toxicology Technical Committee (GTTC) analyzed data from the National Toxicology Program (NTP) Ames test database to evaluate the need for repeat testing when assessing the mutagenic and carcinogenic potential of chemical compounds. Key Findings: High reproducibility for initial positive and…
Botanical Safety Consortium Summit 2024
Thursday October 10 and Friday October 11 2024 In-person: The National Institute of Environmental Health Sciences (NIEHS), Durham, NC, USA Virtual: TBA The Botanical Safety Consortium (BSC) will hold it’s first in-person meeting (with virtual option) at NIEHS in Durham, NC in October 2024. The in-person meeting is limited to members, with preference for steering…
New publication: Advancing botanical safety
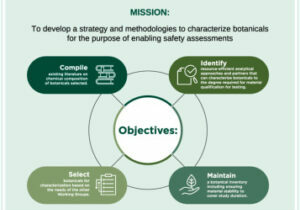
Advancing botanical safety: A strategy for selecting, sourcing, and characterizing botanicals for developing toxicological tools. Waidyanatha et al., February 2024. Food and Chemical Toxicology. https://doi.org/10.1016/j.fct.2024.114537 Increases in botanical use, encompassing herbal medicines and dietary supplements, have underlined a critical need for an advancement in safety assessment methodologies. This manuscript highlights: 1) Botanical Safety Consortium’s strategy for…
New publication in Food and Chemical Toxicology
Neuroactivity screening of botanical extracts using microelectrode array (MEA) recordings Regina G.D.M. van Kleef, Michelle R. Embry, Constance A. Mitchell, Remco H.S. Westerink. Food and Chemical Toxicology. https://doi.org/10.1016/j.fct.2024.114438 The HESI Botanical Safety Consortium collaborates to develop screening strategies that can efficiently identify botanical-induced toxicity. The study presented here evaluates the applicability of in vitro multi-well microelectrode…
International Congress on Natural Products Research (ICNPR)
13 – 17 July 2024 The Botanical Safety Consortium is holding a training course as part of the meeting on Saturday 13 July entitled “Cultivating Safety: Toxicology 101 of Botanicals and Natural Products.“ This course will offer a deep dive into the world of botanicals, bridging the gap between their traditional uses and modern applications in supplements,…
ICSB 2024
15 -18 April 2024 The Botanical Safety Consortium will be in attendance and presenting at the 22nd International Conference on the Science of Botanicals. Speakers include Connie Mitchell (HESI), and committee members Cynthia Rider (NIEHS), Amy Roe (P&G), Bill Gurley (U of Mississippi), and Holly Johnson (AHPA).
34th German Society for Environmental Mutation Research Meeting (GUM)
20 – 22 March 2024 The Botanical Safety Consortium will present two posters at the 34th German Society for Environmental Mutation Research Meeting (GUM). When: 20 – 22 March, 2024 Where: Kaiserslautern, Germany A Strategy for Developing a Robust Framework of Genotoxicity Assays for Safety Assessment of Botanicals The Botanical Safety Consortium: Collaborative Effort to…
SOT 2024
10 – 14 March 2024 The BSC will be presenting at SOT 2024 in Salt Lake City, UT, USA. Monday, March 11@1:45-4:30pm Symposium Session: Botanical-Induced Toxicity: Liver Injury and Botanical Drug Interactions, chaired by Amy Roe (P&G). BSC speakers include Steve Ferguson (NIEHS), Philip Yeager (US FDA), Mary Paine (Washington State University), Igor Koturbash (university Arkansas…
Botanical Safety Consortium: Introduction and Updates
18 January 2024 Connie Mitchell, Michelle Embry & Julie Krzykwa will be presenting to the US FDA Center for Food Safety and Applied Nutrition (CFSAN) to update the progress of the HESI Botanical Safety Consortium.
The Botanical Safety Consortium (BSC) was officially convened in November 2019, as the result of a Memorandum of Understanding between the US Food and Drug Administration (FDA), the National Institutes of Health’s National Institute of Environmental Health Sciences (NIEHS), and the non-profit Health and Environmental Sciences Institute (HESI).
Get Involved
At a Glance
Learn More
Our current charge is to evaluate the suitability of assays for botanicals as complex mixtures.
Latest News
A public-private partnership to enhance the botanical safety toolkit
Botanical dietary supplement and herbal medicine use is increasing both in the United States and worldwide. Products made from botanicals can be variable in chemical composition, with many…
Botanical Safety Consortium Annual Meeting 2021
BSC 2021 Virtual Annual Meeting June 2021 Welcomes & Objectives (Connie Mitchell, HESI) Botanical Safety Consortium Introduction & Updates (Dr. Cara Welch, US FDA) Keynote 1: The hERG Screen Project:…
Session at ICSB 2021
20th Annual Oxford ICSB March 28th – 30th, 2022 The Oxford International Conference on the Science of Botanicals is an annual meeting to discuss approaches for post market…
2020 Virtual SOT Session
“Applying Modern Toxicology to Botanical Dietary Supplements” Click to access PDFs of the presentations: Applying Modern Toxicology to Botanical Dietary Supplements. Michelle Embry (HESI, Washington, DC) Chemical Analysis: The…
Botanical researchers aim to fill gap in safety testing
Of the approximately 28,000 plants that have medicinal properties, only a few have been assessed for safety. Scientists discussed an ambitious effort to fill that gap at the first public…
Botanical Safety Taken on by New Consortium (NIH Record)
Looking to address concerns about the safety of botanicals, NIEHS joined forces with FDA and the nonprofit Health and Environmental Sciences Institute (HESI) to form the Botanical Safety…
Botanical safety taken on by new consortium
Looking to address concerns about the safety of botanicals, NIEHS joined forces with the U.S. Food and Drug Administration (FDA) and the nonprofit Health and Environmental Sciences Institute (HESI) to…
FDA: Botanical Safety Consortium Formally Convened
Silver Spring, MD-The U.S. Food and Drug Administration (FDA) announced in a constituent update that the Botanical Safety Consortium (BSC) has formally been convened. Calling it a “milestone,” FDA said…
FDA convenes the Botanical Safety Consortium
The U.S. Food and Drug Administration (FDA) has announced that the Botanical Safety Consortium (BSC) has formally been convened. This milestone is the result of a Memorandum of…